In a head-to-head comparison, F-18 fluroestradiol (FES)-PET/CT was better than standard FDG-PET/CT for the initial staging of estrogen receptor (ER)-positive breast cancer patients, according to a study published on December 18 in the European Journal of Hybrid Imaging.
In a retrospective analysis, a team in Bangkok, Thailand, found that F-18 FES PET scans detected more metastatic disease in patients than F-18 FDG-PET, with the authors ultimately suggesting the use of both radiotracers for the most accurate staging.
“F-18 FES-PET/CT could detect true metastatic lesions better than F-18 FDG-PET/CT,” noted first author Peerapon Kiatkittikul, MD, of the Chulabhorn Royal Academy’s National Cyclotron and PET Centre.
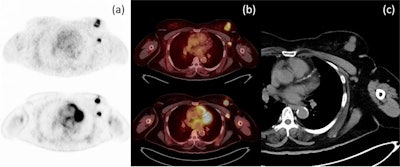
Axial PET (a) and axial fusion (b) images of F-18 FES (top) and F-18 FDG (bottom) with concordant uptake of both radiotracers in a left breast mass and left axillary node metastasis in correlation with CT imaging (c). Image courtesy of the European Journal of Hybrid Imaging.
F-18 FDG is the most widely used PET radiotracer for evaluating various cancers, including breast cancer, the researchers noted. Conversely, F-18 FES-PET (Cerianna, GE HealthCare) was approved in the U.S. in 2020 for clinical use in patients with ER-positive cases as an adjunct to biopsy. Researchers are conducting ongoing work to define the clinical scenarios where the tracer may be most helpful, the authors noted.
To that end, in this study, the group compared the diagnostic performance of F-18 FDG and F-18 FES-PET/CT in the initial staging of ER-positive breast cancer. The group culled imaging from 28 breast cancer patients who underwent both scans for initial staging within two weeks at Chulabhorn Hospital in Bangkok between September 2020 and October 2022. Inclusion criteria were patients age 18 and older with pathologically confirmed cases.