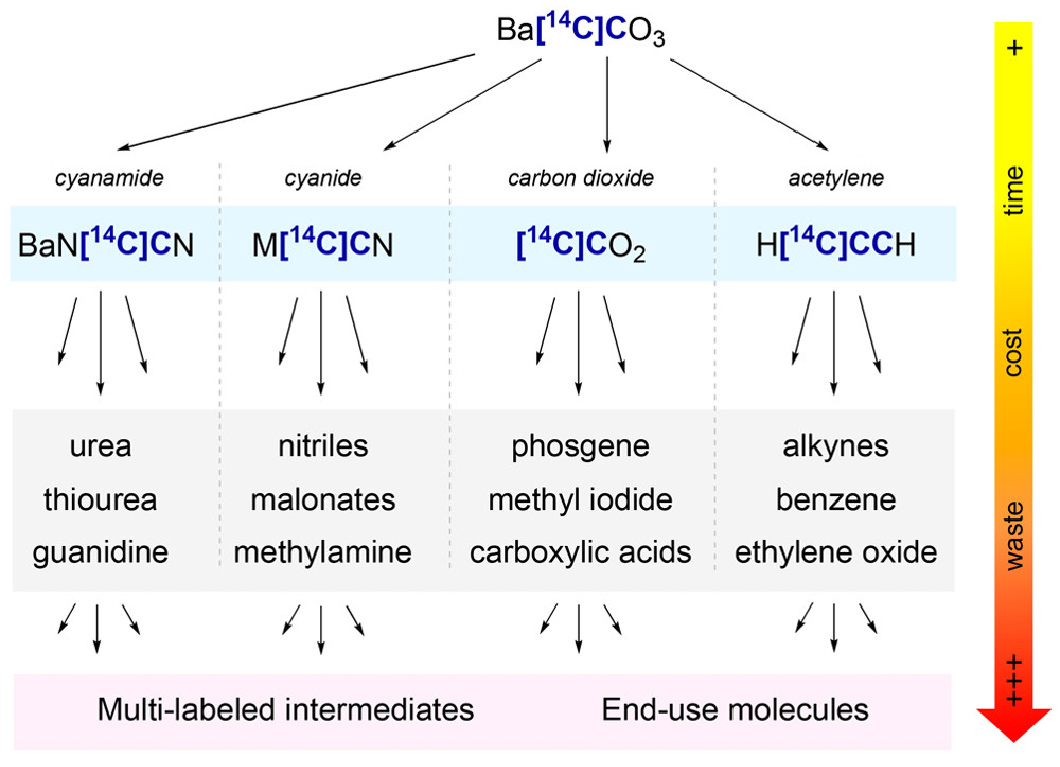
Labeled synthesis of the tested compounds: Preferred markers can be designed according to the chemical structure, synthesis process and early metabolite identification results.

Basic Technical Date
| Chemical Form | Barium [14C] carbonate (Ba[14C]CO3) |
Physical For | Dry powder |
Specific Activity | ① Minimum 280mCi/g ② Minimum 226mCi/g |
| Radioisotopic Purity | Minimum 99.9% as a fraction of C-14 |
Chemical Purity | Minimum 97% BaCO3 by mass |
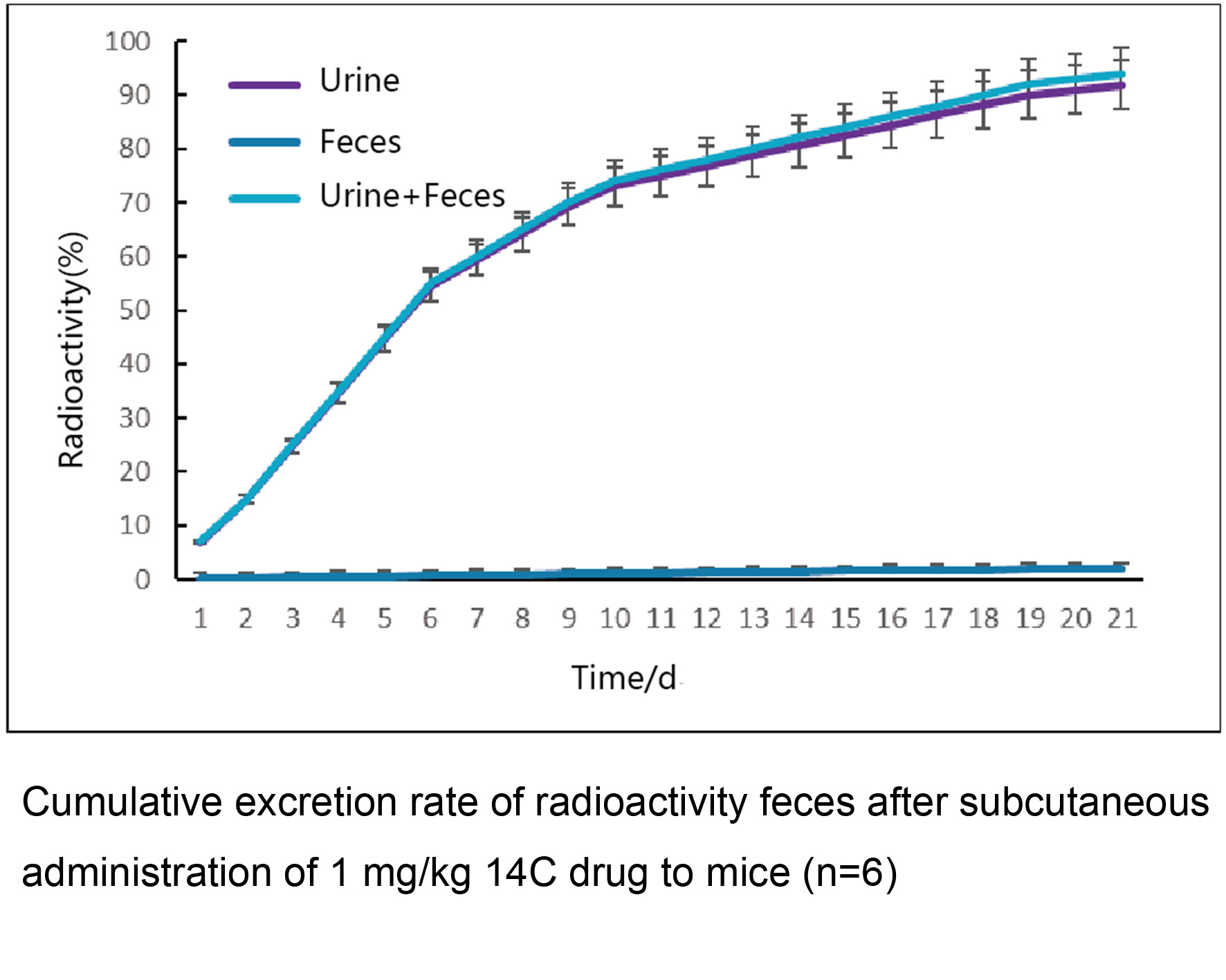
■ Recovery, excretion and mass balance results of total radioactivity in urine, feces, and bile
■ Distribution and elimination of the total radioactivity in the designated tissues
■ Tissue distribution of total radioactivity in mice or rats through quantitative whole-body autoradiography
■ Plasma concentration-time profiles for total radioactivity in whole blood
■ Drug metabolite profiling and identification
■ Total radioactivity concentration-time profiles in plasma and whole blood;
■ Plasma concentration-time profiles for non-radiolabeled drugs, including the parent drugs and metabolites;
■ Statistics of pharmacokinetic parameters of total radioactivity, parent drug and metabolites in plasma;
■ Excretion rate-time profiles of radioactivity in urine, feces, and total excretion;
■ Quantification of radioactivity associated with parent drug and identified metabolite in each collection matrix (such as plasma, urine, feces, etc);
■ Biotransformation pathways of metabolite structures or analysis of metabolites